After four years of research and validation, GemPharmatech’s independently developed fully antibody gene humanized mouse model, NeoMab™, is officially entering the market to serve the therapeutic antibody development needs of biotechnology companies and pharmaceutical enterprises.
The NeoMab™ mouse is developed on the widely used BALB/c genetic background, making it highly suitable for antibody discovery in the industry. It retains the native mouse constant region encoding genes while incorporating human variable gene repertoires at the endogenous loci. (Fig 1). This strategy results in a powerful model that combines the strengths of the mouse immune system with human-specific variable regions, making it ideal for advancing antibody discovery and research endeavors.
Figure 1. Humanization strategy of the NeoMab™ model
Based on in vitro and in vivo experiments, the NeoMab™ mouse model demonstrates the following advantages:
- Close Resemblance to Human Immune Response:NeoMab™ mice utilize human V(D)J genes to encode antibodies, exhibiting gene usage frequency and sequence diversity that closely resemble those of humans.
- Intact Immune System: The immune system of NeoMab™ mice remains intact, with proportions of various immune cell subsets similar to those of the BALB/c mice.
- Normal B-Cell Development:NeoMab™ mice exhibit normal antibody class switching, somatic hypermutation, and B-cell development, with comparable levels of immunoglobulin in their serum to BALB/c background mice.
- Robust immune response:After immunization with human recombinant protein antigens, NeoMab™ mice display antigen-specific serum titers similar to BALB/c mice, reaching approximately 105-106 after three immunizations.
- High Affinity: Antibodies obtained from NeoMab™ mice demonstrate an affinity of approximately 10-10 to 10-8 (as determined by SPR detection), which is comparable to or even higher than that of FDA-approved therapeutic antibody drugs.
- Effective in vitro and in vivo Functionality: Thein vitro functional activity and in vivo efficacy of NeoMab™-derived antibodies closely resemble those of FDA-approved therapeutic antibody drugs.
GemPharmatech’s NeoMab™, a fully antibody gene humanized model, along with its advanced technical platform, not only facilitates but expedites the preclinical discovery and validation processes for forward-thinking pharmaceutical enterprises. By leveraging licensing agreements and high-throughput screening platforms, GemPharmatech empowers these companies to achieve their objectives swiftly and economically. This approach optimizes capital investment while catalyzing innovative drug development endeavors, ultimately paving the way for groundbreaking advancements in the field.
Providing Comprehensive Support for Drug Development and Research
GemPharmatech exemplifies dedication in furnishing mouse models and support services for the advancement of disease mechanism research, pharmaceutical development, and translational studies. At present, we have established extensive repositories of model resources, encompassing KOAP mice and tool mice for drug screening purposes. These invaluable resources bestow essential support for target validation and pharmacological efficacy investigations. Moreover, our research service platforms have been meticulously developed in prominent domains such as oncology, metabolism, cardiovascular sciences, immunology, and neuroscience. By harnessing the synergistic potential of these platforms in conjunction with the advantages of our model resources, we are capable of delivering comprehensive non-clinical research services across diverse fields.
NeoMab™, our fully antibody gene humanized model, serves as a pivotal addition to the GemPharmatech family. This innovative platform augments antibody research and discovery endeavors by integrating with our expansive array of model resources and professional technical service platforms. Through this harmonious fusion, we empower drug development enterprises with comprehensive support, propelling the process of uncovering and advancing new therapeutic agents. Our objective is to translate ideas into tangible solutions, foster collaborative partnerships, and achieve mutual triumphs in the realm of scientific progress and enhanced healthcare outcomes.
About the fully antibody gene humanized model
Since the FDA’s landmark approval of the pioneering therapeutic antibody OKT3 in 1986 (Ecker, Jones et al., 2015), the realm of therapeutic antibodies has undergone rapid and consequential advancements, establishing itself as a pivotal component of modern biopharmaceuticals. Notably, these antibodies have ascended to become one of the most commercially successful classes of drugs in recent years. However, the development of antibody-based medications continues to face challenges such as low success rates, high costs, and protracted timelines. Particularly concerning for antibody therapeutics, which are classified as large-molecule drugs, is the presence of anti-drug antibodies (ADA). Predicting the ADA formation risk of an antibody in the preclinical stage is difficult.
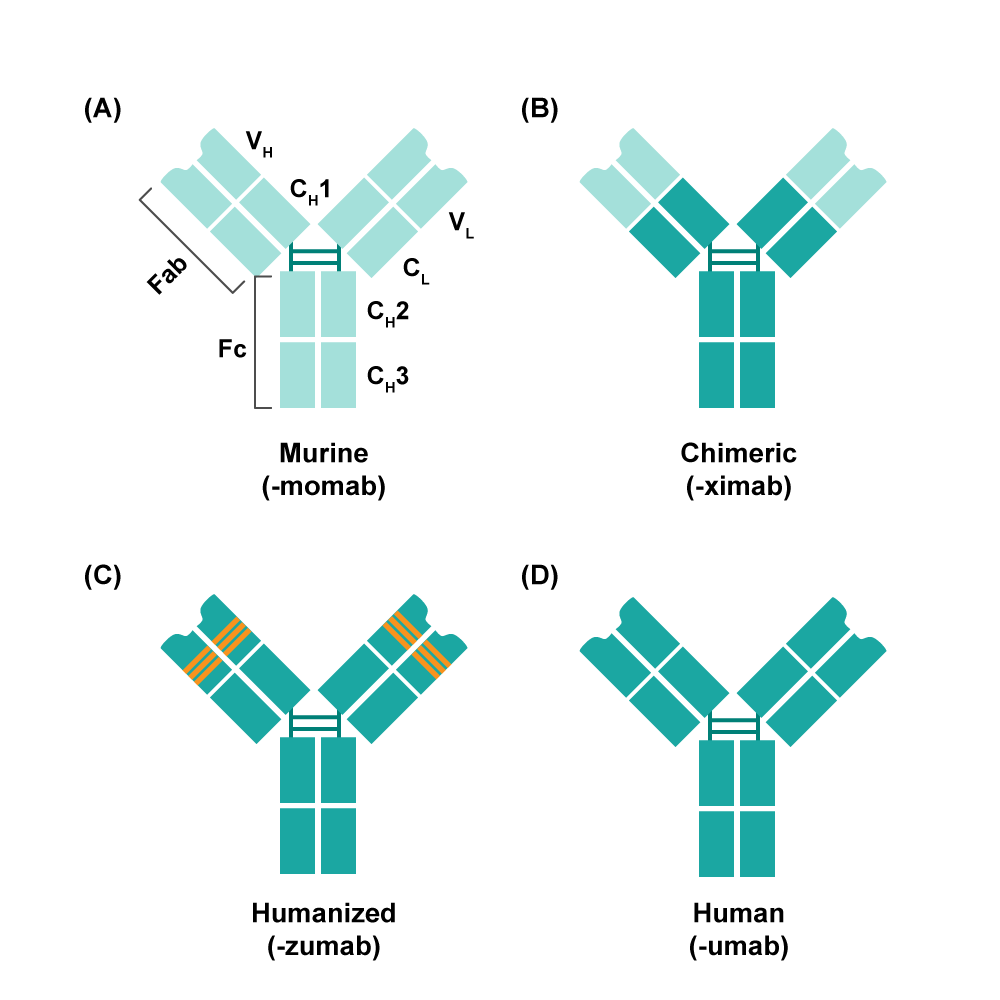
Figure 2.Schematic overview of antibody humanization from different antibodies (Lu et al. Journal of Biomedical Science. 2020)
In an effort to reduce ADA, therapeutic antibodies have been developed in stages, starting with murine antibodies and moving on to chimeric antibodies, engineered antibodies, humanized antibodies, and finally fully human antibodies (Lu, Hwang, et al., 2020) (Fig 2). Extensive research and development practices have indicated that an increased proportion of human sequences within these antibodies correlates with a decreased risk of ADA (Safdari, Farajnia et al., 2013). Nevertheless, it is essential to acknowledge that the process of humanization incurs additional costs and time, and even humanized antibodies cannot entirely eliminate the potential for ADA. For example, in the case of antibodies targeting PCSK9, the fully human antibodies Alirocumab and Evolocumab, derived from transgenic mice, have successfully obtained approval and been launched on the market. In contrast, Bococizumab failed to progress beyond phase III clinical trials due to its heightened ADA (Ridker, Tardif et al., 2017). In comparison, fully human antibodies encoded exclusively by human sequences offer distinct advantages in development.
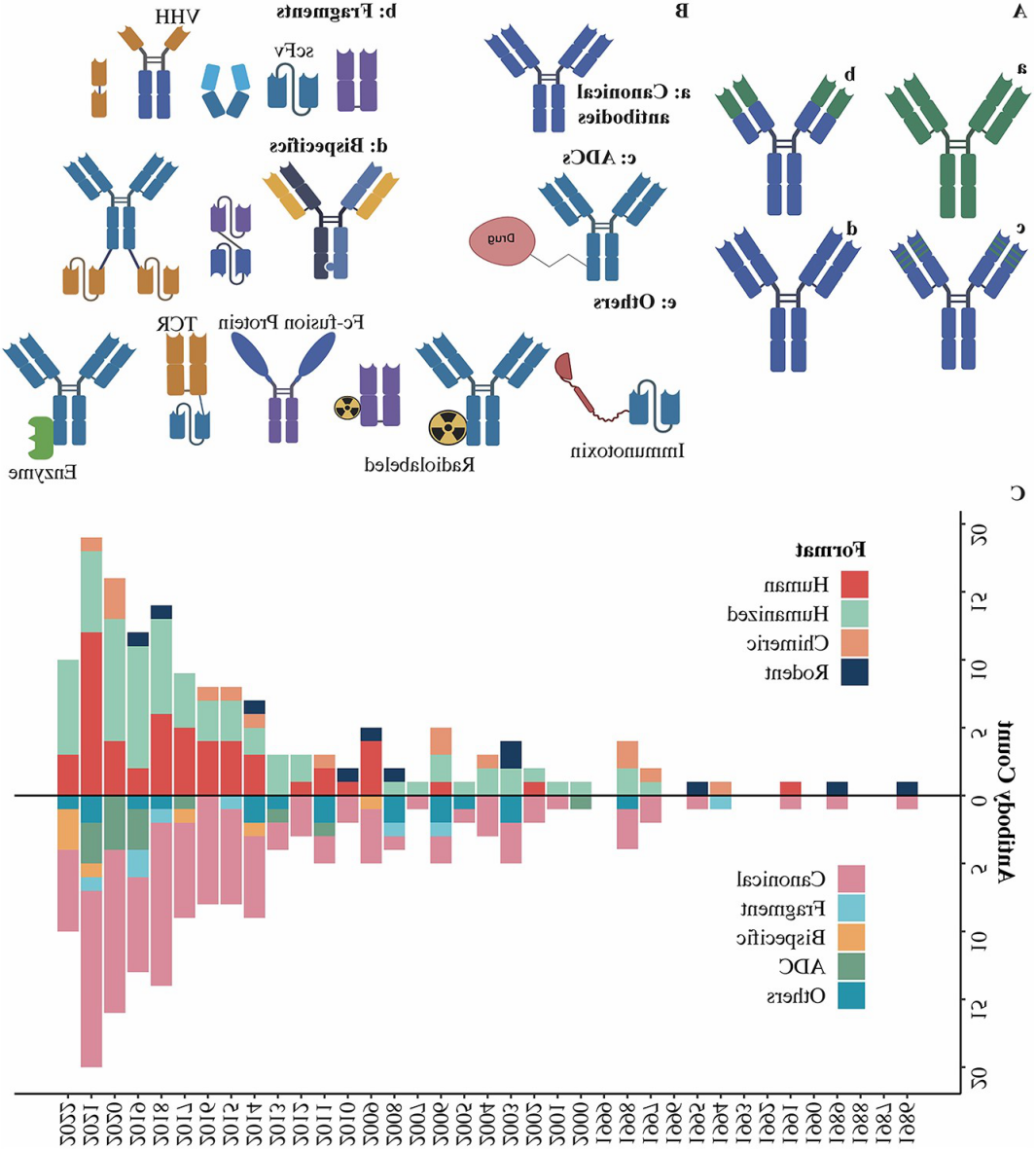
Figure 3. Antibody discovery and engineering for approved antibodies (Lyu, Xiaochen, et al. Antib Ther. 2022)
By 2022, over 160 antibody therapies had received approval for commercial use, with a notable inclination toward the dominance of fully human antibodies (Lyu, Zhao et al., 2022). Among the top 50 antibody drugs in terms of sales during that year, fully human antibodies accounted for 45% of the total. Furthermore, within the realm of approved fully human antibodies, an impressive 70% were derived from transgenic mice. This unequivocally demonstrates the viability and advantages of transgenic mice as a platform for the development of fully human antibodies.
The first generation of fully antibody gene humanized models was achieved through “TG+KO” technology. This was done by using genetic engineering to make transgenic mice (TG mice) with human antibody gene segments while knocking out the natural antibody gene at the same time. Noteworthy platforms arising from this era include the HuMab platform (Lonberg, Taylor et al., 1994; Taylor, Carmack et al., 1994), established in 1994, as well as the XenoMouse platform (Jakobovits, 1995), developed in 1997. Although these models possess limitations concerning the number of human gene segments and differences in immune responses compared to wild-type mice, they have nonetheless contributed to two-thirds of the approved fully human antibodies derived from transgenic mice (19 antibodies).
The subsequent generation of models employs site-specific knock-in techniques, wherein the human antibody variable region (V) gene library is introduced into the endogenous antibody gene locus of the mouse while still retaining the mouse constant region gene segments. This strategy, also embraced by the NeoMab™ platform, overcomes the limitations associated with human gene segment numbers and preserves the regulation of endogenous expression and Fc-mediated signaling (Murphy, Macdonald et al., 2014), resulting in an immune response closely resembling that of wild-type mice. Notably, one of the most renowned platforms in this domain is Regeneron’s VelocImmune platform (Macdonald, Karow et al., 2014; Murphy, Macdonald et al., 2014), established in 2009, which has hitherto yielded seven approved antibodies.
Visit website to learn more: https://en.gempharmatech.com/content/details100032_3899406.html
References:
[1] Ecker, D. M., S. D. Jones and H. L. Levine (2015). “The therapeutic monoclonal antibody market.” MAbs 7(1): 9-14.
[2] Jakobovits, A. (1995). “Production of fully human antibodies by transgenic mice.”Curr Opin Biotechnol 6(5): 561-566.
[3] Lonberg, N., L. D. Taylor, F. A. Harding, M. Trounstine, K. M. Higgins, S. R. Schramm, C. C. Kuo, R. Mashayekh, K. Wymore, J. G. McCabe and et al. (1994). “Antigen-specific human antibodies from mice comprising four distinct genetic modifications.” Nature 368(6474): 856-859.
[4] Lu, R.-M., Y.-C. Hwang, I. J. Liu, C.-C. Lee, H.-Z. Tsai, H.-J. Li and H.-C. Wu (2020). “Development of therapeutic antibodies for the treatment of diseases.” Journal of Biomedical Science 27(1): 1.
[5] Lyu, X., Q. Zhao, J. Hui, T. Wang, M. Lin, K. Wang, J. Zhang, J. Shentu, P. A. Dalby, H. Zhang and B. Liu (2022). “The global landscape of approved antibody therapies.” Antib Ther 5(4): 233-257.
[6] Macdonald, L. E., M. Karow, S. Stevens, W. Auerbach, W. T. Poueymirou, J. Yasenchak, D. Frendewey, D. M. Valenzuela, C. C. Giallourakis, F. W. Alt, G. D. Yancopoulos and A. J. Murphy (2014). “Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes.” Proc Natl Acad Sci U S A 111(14): 5147-5152.
[7] Murphy, A. J., L. E. Macdonald, S. Stevens, M. Karow, A. T. Dore, K. Pobursky, T. T. Huang, W. T. Poueymirou, L. Esau, M. Meola, W. Mikulka, P. Krueger, J. Fairhurst, D. M. Valenzuela, N. Papadopoulos and G. D. Yancopoulos (2014). “Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice.” Proc Natl Acad Sci U S A 111(14): 5153-5158.
[8] Ridker, P. M., J. C. Tardif, P. Amarenco, W. Duggan, R. J. Glynn, J. W. Jukema, J. J. P. Kastelein, A. M. Kim, W. Koenig, S. Nissen, J. Revkin, L. M. Rose, R. D. Santos, P. F. Schwartz, C. L. Shear, C. Yunis and S. Investigators (2017). “Lipid-Reduction Variability and Antidrug-Antibody Formation with Bococizumab.” N Engl J Med 376(16): 1517-1526.
[9] Safdari, Y., S. Farajnia, M. Asgharzadeh and M. Khalili (2013). “Antibody humanization methods – a review and update.” Biotechnol Genet Eng Rev29: 175-186.
[10] Taylor, L. D., C. E. Carmack, D. Huszar, K. M. Higgins, R. Mashayekh, G. Sequar, S. R. Schramm, C. C. Kuo, S. L. O’Donnell, R. M. Kay and et al. (1994). “Human immunoglobulin transgenes undergo rearrangement, somatic mutation and class switching in mice that lack endogenous IgM.” Int Immunol 6(4): 579-591.

